Lioness Medtech säljer och servar ledande medtech inom diagnostik och behandling av cancer och degenerativa sjukdomar såsom Alzheimers och ALS.
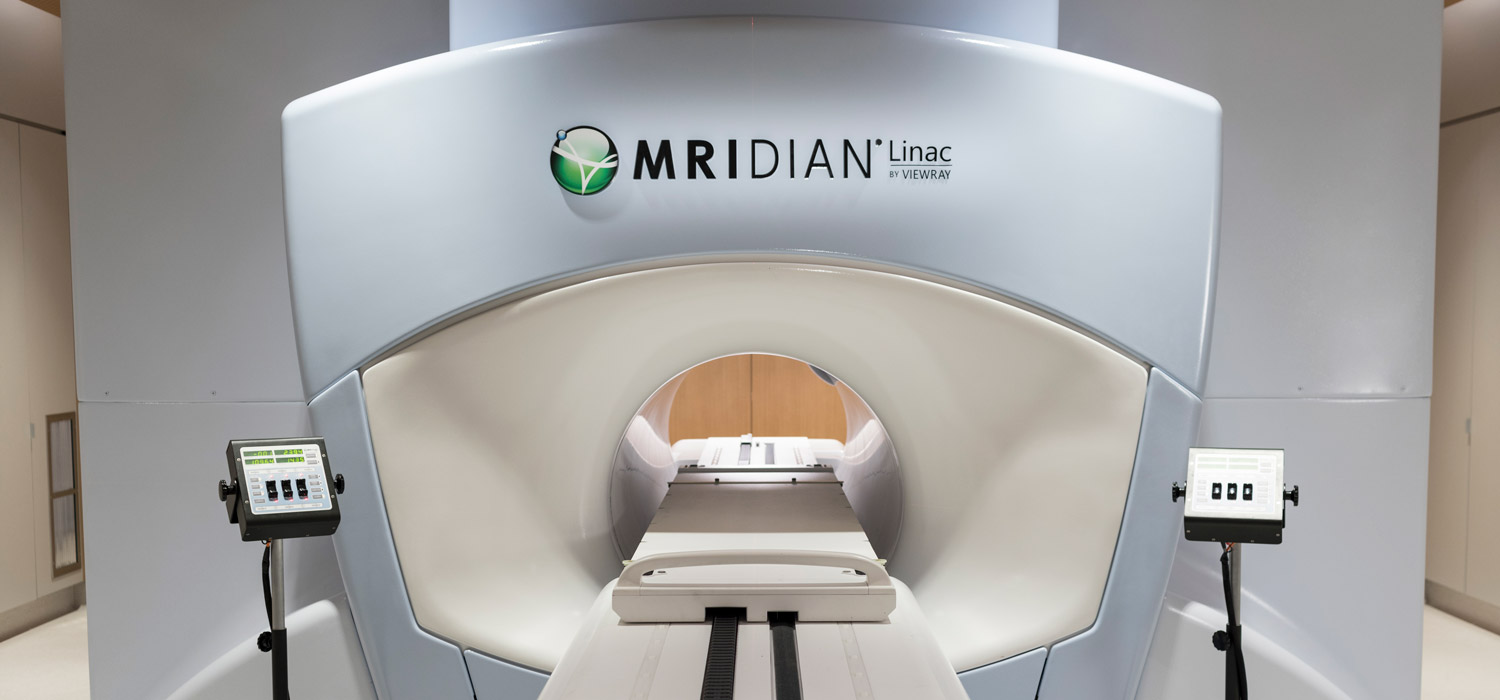
Lioness Medtech utför service av flera MRIdian linac system i norra Europa. Vi kan erbjuda olika nivåer av service, på plats och på distans. Vi servar även cyklotroner från Sumitomo.
Tveka inte att maila oss på info@lionessmedtech.com för mer information.
Läs mer om den senaste forskningen och tekniken i utvecklingens framkant på lionessmedtech.com.
Lioness Medtech provides and services leading medtech in diagnostics and treatments of cancer and neurological diseases.
Our expert service team assist several hospitals in Europe with on-site and remote monitoring, preventive maintenance and repairs as needed. Lioness offers expert service for accelerators and imaging equipment.
Don't hesitate to contact us at info@lionessmedtech.com for more information.
Read the about the latest research and technology on lionessmedtech.com.